


Next: Continuum
Up: (Sub-)mm radiative transfer
Previous: (Sub-)mm radiative transfer
Contents
Under clear weather, the atmospheric absorption/emission at
(sub)-millimeter wavelengths is dominated by the rotational and fine
structure line of molecules in their ground electronic and low vibrational
states. Three molecules plays a major role
-
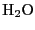
- is the most abundant polar molecule present in the
atmosphere. Its electric dipole moment is 1.88 Debye. ATM 2009 also takes
into account the water isotopomers.
-
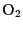
- Being an homonuclear molecule,
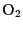 has a zero-valued
electric dipole moment. However, it has a triplet electronic ground
state, with two electrons paired with parallel spins, implying a weak
magnetic dipole moment. The weakness of this dipole moment is compensated
by the large
has a zero-valued
electric dipole moment. However, it has a triplet electronic ground
state, with two electrons paired with parallel spins, implying a weak
magnetic dipole moment. The weakness of this dipole moment is compensated
by the large
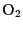 abundance, implying
abundance, implying
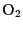 line intensities similar to
line intensities similar to
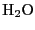 ones.
ones.
-
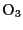
- is an asymmetric top molecule, such as
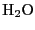 . Despite the
small ozone abundance, its lines have significant peak opacities,
especially in the submillimeter domain. This is dues to linewidths much
narrower than water. This can be explained by two facts: 1) the ozone
dipole moment is 0.53 Debye, about 3 times less than water and 2) ozone
is mostly concentrated between 11 and 40 km of altitude, implying
linewidths 20 times narrower than sea level lindwidths.
. Despite the
small ozone abundance, its lines have significant peak opacities,
especially in the submillimeter domain. This is dues to linewidths much
narrower than water. This can be explained by two facts: 1) the ozone
dipole moment is 0.53 Debye, about 3 times less than water and 2) ozone
is mostly concentrated between 11 and 40 km of altitude, implying
linewidths 20 times narrower than sea level lindwidths.



Next: Continuum
Up: (Sub-)mm radiative transfer
Previous: (Sub-)mm radiative transfer
Contents
Gildas manager
2014-07-01